Alzheimer’s disease (AD) has long eluded a cure, causing researchers to delve deeper into the biological underpinnings of the disorder for new, inventive, and multi-factorial strategies to reduce neurodegeneration before and after onset. One investigational treatment provided by Athira, called ATH-1017, recently began Phase II clinical trial enrollment with our clinic. If this blog peaks your interest, please feel free to reach out to us and see if you or someone you know might be applicable for involvement in the trial.
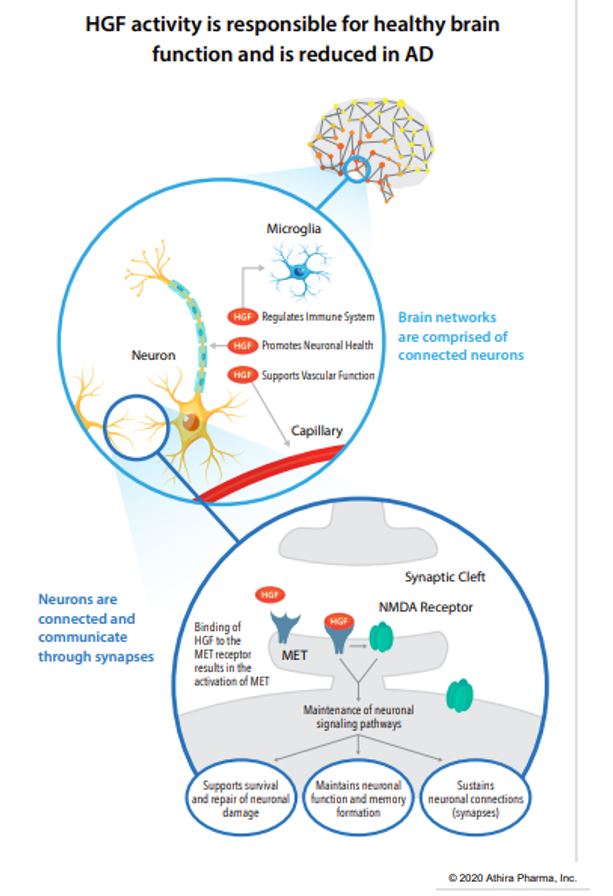
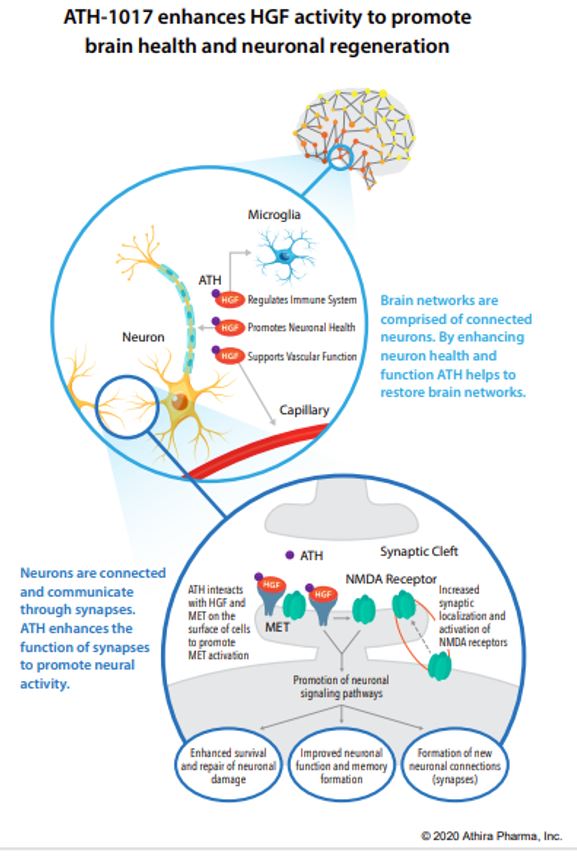
ATH-1017 is a Hepatocyte Growth Factor (HGF) Receptor Agonist, meaning that administration simulates the presence of HGF, activating a kinase receptor-protein called MET. The HGF/MET complex is a neurotrophic factor meaning, when functioning properly, it protects neurons from degeneration and may induce regeneration of lost neuronal connections in dysfunctioning brain regions (such as the hippocampus in AD). Patients with AD have reduced hippocampal MET receptors. ATH-1017 targets the HGF/MET complex in order to enhance the neuroprotective CNS effects.
In animal studies the investigational drug improved learning and memory in aged rats, prevented motor symptoms and neuronal loss in rat models of Parkinson’s disease (PD), and stimulated dendritic arborization and synaptogenesis. Furthermore, in humans during a Phase I trial it improved brain activity as measured by gamma power and p300 latency in participants with AD (both measures of increased learning, memory, executive functioning, and processing speed). It also produced a dose-related increase in gamma power in healthy controls suggesting that ATH-1017 may have wide therapeutic effects outside of just AD and PD. Furthermore, in all dosage groups of the Phase I trial, no investigational-drug-related adverse events were recorded, meaning the therapeutic dose required is very safe. Cognitive testing is now added to the new trial in order to assess clinical efficacy.