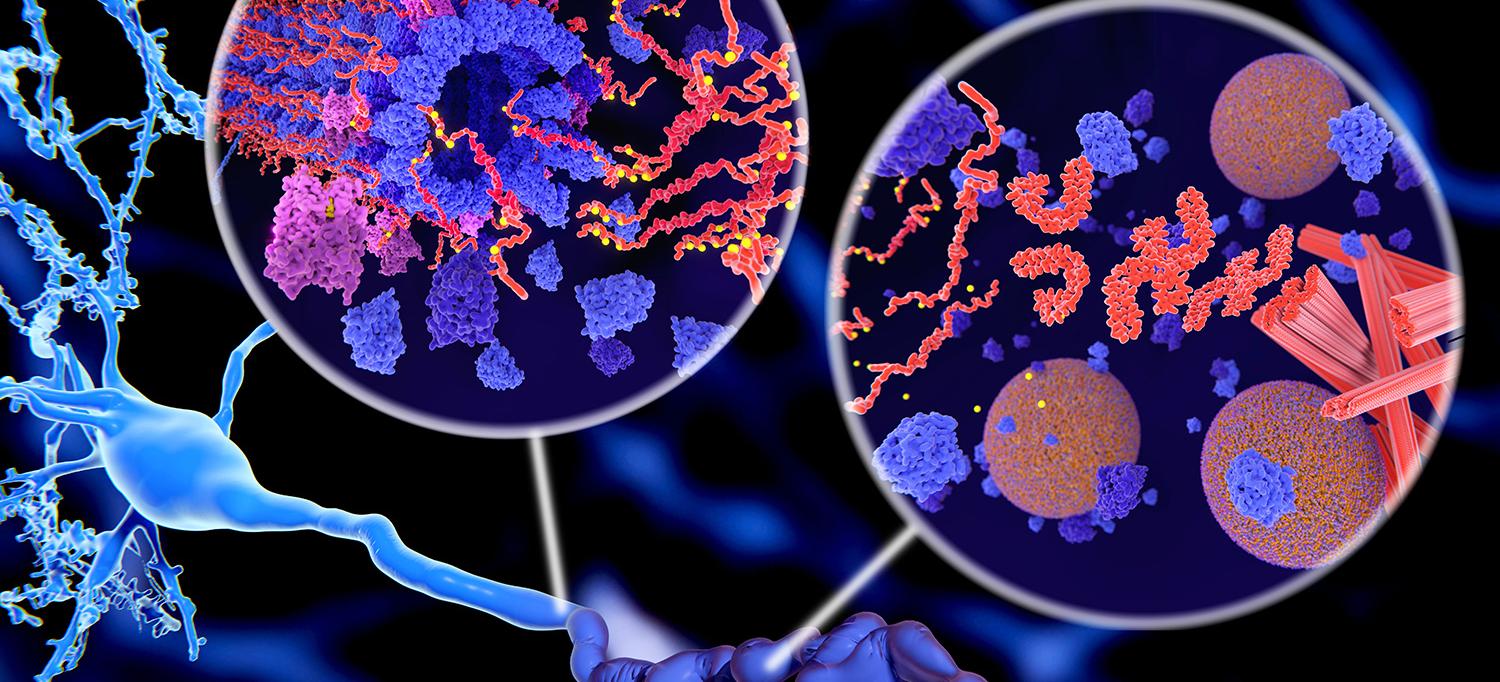
Insulin is typically associated with regulating blood glucose levels and diabetes, but it also serves as a crucial signaling molecule throughout the body, including the central nervous system (CNS). In fact, there is evidence that insulin may play a role in the development of Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI). Dysregulation of insulin signaling negatively impacts cognition and increases deposition of amyloid plaques and tau tangles. Luckily, there are also mechanisms that can upregulate insulin sensitivity and these treatments have potential to reduce or alleviate AD symptoms.
Insulin is a hormone released primarily by the pancreas. It can cross the blood-brain barrier (BBB) to have CNS effects. However, if one develops insulin resistance, which is correlated with AD, normal tissues fail to sufficiently respond to the presence of insulin. In these cases, insulin dysregulation induces hyperglycemia, too much sugar in the blood, which leads to glucose neurotoxicity, reduced cerebral blood flow, and accumulation of toxic byproducts in the brain, all of which can lead to cognitive impairment.
There are several ways to test insulin resistance but they all follow a basic method of administering insulin and monitoring the response of the target tissue, whether peripheral or in the CNS. There is a close bi-directional relationship between insulin functioning in the body and the brain. For patients with AD there is a trend towards insulin insensitivity and hyperinsulinemia (too much insulin in the blood). Excess insulin downregulates the receptors that move insulin through the BBB into the CNS, meaning less insulin in the brain.
A reduction in CNS insulin is significant because insulin in the brain protects against amyloid-beta synaptotoxicity and promotes clearance of plaques. AD patients with peripheral insulin resistance have increased amyloid deposition compared to healthy controls. Tests of peripheral insulin resistance successfully predict amyloid deposition in the brain 15 years later, as confirmed by an amyloid PET scan. Furthermore, patients with altered insulin signaling from diabetes have increased tau levels in cerebrospinal fluid. A final additive risk factor is that excess insulin acts as a vasoconstrictor limiting blood flow to the brain and decreasing amyloid and tau clearance. Dysfunctional insulin signaling may be a risk factor for AD.
Understanding how insulin resistance impacts AD risk has improved our range of potential treatments. Intranasal insulin administration allows it to bypass the BBB and enter the CNS, reducing AD pathology and improving memory in rats after long-term administration. In humans, twenty-one days of treatment enhanced episodic memory. However, clinical trials testing intranasal insulin show mixed results indicating the need for further research. One can also increase insulin sensitivity with “insulin sensitizers”, such as Metformin, but evidence for these treatments in AD is limited. In mice and primates a GLP-1 agonist, which stimulates insulin production and regulates glucostasis, called liraglutide preserved memory and increased hippocampal neuronal density. Liraglutide is currently in a Phase II clinical trial for use in humans with AD.
Although these potential treatments are promising there are proven ways to enhance insulin sensitivity and reduce AD risk right now and without a prescription. Consuming a diet of primarily polyunsaturated fatty acids, nuts, and plant-based foods correlates with increased insulin sensitivity and decreased risk of AD-related cognitive decline compared to diets containing higher saturated fats, animal proteins, and refined sugars. Additionally, regular exercise is a powerful modulator of insulin sensitivity and has also been shown to reduce risk of AD. In the meantime, while we wait for the aforementioned therapies to be approved, a lifestyle and diet change is not only protective against AD, but can also improve your overall general health.